Ammonia is a volatile hydrogen compound (hydrogen nitride) that plays a leading role in modern industry.
Although it was discovered only in the eighteenth century, it has been known to man since time immemorial. An aqueous solution of ammonia is ammonia. This substance is found in decomposition products of living organisms and urine. Therefore, when organic matter (the remains of plants, animals) decomposes, ammonia is released, and this gives rise to a pungent smell of rotting (ammonia).
History of ammonia
Ammonia was discovered at the end of the eighteenth century by the British chemist Joseph Priestley, one of the founders of modern chemistry, who also made many important discoveries in other fields of science (physics, biology, optics).
For example, the list of his inventions includes: carbonated water, for which he received a medal from the Royal Society of London, and the well-known eraser (previously, everyone used bread to erase graphite).
There is no denying that Joseph Priestley made enormous contributions to chemistry, especially in the field of gases, but many of his achievements were achieved by accident.
Joseph Priestley prepared ammonia by heating ammonium chloride (ammonia) with calcium hydroxide (slaked lime) and then collecting the resulting gas in a mercury bath.
A mercury bath is a special device created by Priestley to concentrate gases. At room temperature, mercury is a liquid with a high density, which prevents it from absorbing gases. The scientist easily isolated them from substances by heating them over the surface of mercury.
Ammonia equation:
2NH4Cl + Ca(OH)2 = NH3 + CaCl2.
After the discovery of ammonia by Joseph Priestley, its study did not stand still.
In 1784, the composition of this substance was established by the chemist Louis Berthollet, who decomposed it into its original elements by electric discharge.
It received the name “ammonia” already in 1787 from the Latin name for ammonia, and the name “ammonia” itself, which we are accustomed to using, was introduced by Yakov Dmitrievich Zakharov in 1801.
But here's what's interesting. A hundred years before Joseph Priestley and his discovery of ammonia, scientist Robert Boyle observed a phenomenon in which a stick, previously soaked in hydrochloric acid, began to smoke when it was brought to the gas released by burning manure. This is explained by the fact that the acid and ammonia reacted, and its products contained ammonium chloride, the particles of which created the smoke. It turns out that ammonia was discovered by experimental methods a long time ago, but its presence in the world was proven much later.
Methods of obtaining
About 100 million tons of ammonia are produced annually in the world, so this process can rightfully be considered one of the most important in the world. It is produced in liquid form or as a twenty-five percent solution.
There are the following ways to obtain it:
1. In industry, ammonia is produced through the synthesis of nitrogen and hydrogen, which is accompanied by the release of heat. Moreover, this reaction can only take place at high temperature, pressure and in the presence of a catalyst, which, while accelerating a weak reaction, does not itself enter into it.
Ammonia reaction equation:
N2 + 3H2 ⇄ 2NH3 + Q
2. Ammonia can be obtained during the coking of coal.
In fact, coal does not contain any ammonia, but it contains many organic compounds, which contain nitrogen and hydrogen. And when coal is heated strongly (pyrolysis), these components form ammonia, which comes out as a by-product.
3. In the laboratory, ammonia is produced by heating ammonium chloride and calcium hydroxide:
2NH4Cl + Ca(OH)2 → CaCl2 + 2NH3↑ + 2H2O
4. Or by heating ammonium chloride with concentrated alkali:
NH4Cl + NaOH = NaCl + NH3↑ + H2O
What it is?
The main name is ammonium hydroxide, in the international version - Ammonium Hydroxide. It is a weak compound made by mixing ammonia with water molecules. Mentioned in GOST R 53045-2008, clause 87.
Synonymous names:
- E 527 (E–527), European code;
- ammonia hydrate;
- ammonium oxide hydrate;
- ammonia water;
- ammonia, aqueous;
- caustic ammonia;
- ammonium hydroxide;
- ammonia;
- in France - hydroxide de ammonium;
- in Germany - Ammoniumhydroxide.
Kinds
- For the food, medical and cosmetic industries.
- For the agricultural industry.
Description and properties
| Index | Standard |
| Appearance | Transparent colorless liquid (sometimes yellowish). |
| Compound |
|
| Smell | Sharp and characteristic. |
| Solubility | High. |
| Main substance content | Not less than 27%. |
| Taste | Specific. |
| Density | 0.91 (25% solution) g/cm³. |
| Other |
|
What class does it belong to?
Ammonium hydroxide is an acidity regulator and is a raw material of synthetic origin.
From what and how do they get it?
Additive E527 is obtained by combining purified water and ammonia. For this purpose, the products of burning coal are used, after which they are dissolved in water.
The production process takes place in several stages:
- distillation;
- reflux;
- condensation of ammonia water.
The E527 additive is produced by Russian enterprises:
- JSC Acron (Veliky Novgorod);
- chemical (Tula region);
- OJSC Togliattiazot.
Leading foreign manufacturers:
- Knuchel Farben AG (Germany);
- Cutrin (Finland);
- Lubon Industry Co., Ltd. (China).
Application
Ammonia is an irreplaceable and truly necessary substance, without which the world industry would slow down. Its scope is wide: it is involved in all human production processes, from factories and laboratories to medicine. Its advantages are that it is environmentally friendly and a fairly cheap product.
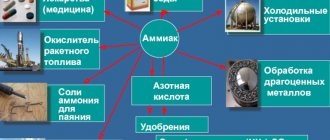
Areas of application of ammonia:
- Chemical industry. It is used in the production of fertilizers, polymers, nitric acid, explosives, and as a solvent (liquid ammonia).
- Refrigeration units. Ammonia evaporates with the absorption of a large amount of heat from the environment, as it has certain thermodynamic properties. Refrigeration systems based on its use are more than efficient, which is why it is the main refrigerant in industry.
- Medicine. Ammonia or a 10% ammonia solution is used to recover from a fainting state (irritation of the receptors of the nasal mucosa helps stimulate breathing), cleaning the surgeon's hands, inducing vomiting, and so on.
- Textile industry. It is used to produce synthetic fibers. Ammonia is also used when cleaning or dyeing various fabrics.
How do you know if a product contains ammonium hydroxide?
The manufacturer displays all information about the presence of this additive on the product label , at the end of the list, along with other acidity regulators, emulsifiers and preservatives. Most often the mark E527 is put, and the decoding is indicated in brackets - ammonium hydroxide.
Sometimes E527 can be found under the name ammonia hydrate or ammonium oxide hydrate. Foreign products use the international name Ammonium Hydroxide or the European code E-527. In medical preparations, the additive is labeled as ammonia.
Physical properties
Here are the physical properties of ammonia:
- Under normal conditions it is a gas.
- Colorless.
- Has a pungent odor.
- Poisonous and very toxic.
- Very soluble in water (one volume of water per seven hundred volumes of ammonia) and a number of organic substances.
- The melting point is -80 °C.
- Boiling point is about -36 °C.
- It is explosive and flammable.
- About half as light as air.
- It has a molecular crystal lattice, therefore it is fusible and fragile.
- The molar mass of ammonia is 17 grams/mol.
- When heated in an oxygen environment, it decomposes into water and nitrogen.
Chemical properties of ammonia
Ammonia is a strong reducing agent, since the degree of oxidation of nitrogen in the molecule is minimal. It is also capable of oxidizing properties, which is much less common.
Reactions with ammonia:
- With acids, ammonia forms ammonium salts, which decompose when heated. With hydrochloric acid, ammonia forms ammonium chloride, and with sulfuric acid, ammonium sulfate.
NH3 + HCL = NH4CL
NH3 + H2SO4 = (NH4)2SO4
- When heated with oxygen, nitrogen is formed, and with the participation of a catalyst (Pt), nitric oxide is obtained.
4NH3 + 5O2 = 4NO + 6H2O
4NH3 + 3O2 = 2N2 + 6H2O
- With water, unstable ammonia hydrate is formed.
NH3 + H2O = NH3 × H2O
Ammonia is capable of exhibiting alkaline properties, therefore, when interacting with water, it forms a weak base - NH4OH. But in fact, such a compound does not exist, so the formula should be written as follows: NH3 × H2O.
- With metal oxides.
2NH3 + 3CuO = 3Cu + N2 + 3H2O
- With halogens.
8NH3 + 3Cl2 =N2 + 6NH4Cl
- With metal salts.
3NH3 + 3Н2О + AlCl3 = Al(OH)3↓ + 3NH4Cl
Ammonia compounds
There are several types of complex substances formed when interacting with ammonia:
- Ammonium salts. They are formed as a result of reactions of ammonia with acids and decompose when heated.
- Amides. These are salts that are obtained by treating alkali metals with ammonia.
- Hydrazine. This is a substance that is obtained by the oxidation of ammonia with sodium hypochlorite in the presence of gelatin.
- Amines. Ammonia reacts with haloalkanes as an addition reaction to form salts.
- Ammonia. With silver and copper salts, ammonia forms complex salts.
Biological role
Ammonia is a substance formed in the organisms of living beings during metabolism, which is a product of nitrogen metabolism in them. It plays an important role in animal physiology, but it is highly toxic to organisms and is almost never found in them in its pure form. Most of it is processed by the liver into a harmless substance - urea or, as it is also called, urea.
It also helps neutralize acids entering the body with food, maintaining the acid-base balance of the blood.
Ammonia is an important source of nitrogen for plants. They mainly absorb it from the soil, but this is a very labor-intensive and inefficient process. Some plants are able to accumulate nitrogen contained in the atmosphere with the help of special enzymes - nitrogenases. After which they process nitrogen into useful compounds, such as proteins and amino acids.
Aggregate states
Ammonia can be in different states of aggregation:
- It is present as a colorless gas with an unpleasant, pungent odor under normal conditions.
- It can also dissolve very well in water, so it can be stored in the form of an aqueous solution with a certain concentration. It liquefies and becomes a liquid as a result of pressure and extreme cooling.
- Ammonia has a solid state in which it appears as colorless cubic crystals.
MORPHOLOGY
Crystals of tetragonal-trioctahedral, rhombic-dodecahedral, less often cubic habit, are usually elongated along L3 or L4 and have a pseudotrigonal or pseudotetragonal appearance. Characterized by elongated skeletal crystals and pseudotrigonal dendrites.
Aggregates – loose earthy masses, fluffy needle-like deposits; crystal druses, stalactites, hollow growths, sinter crusts. drop-shaped forms in niches and caves, fan-shaped, pole-shaped. cellular and parallel-fibrous aggregates; sometimes fills lava pores, cements pieces of lava, and forms the mouths of some fumaroles.
Ammonia poisoning
As mentioned above, ammonia is an extremely toxic and poisonous substance. It is classified as hazard class four.
Poisoning with this gas is accompanied by disruption of many body processes:
- First, the nervous system is affected and the absorption of oxygen by nerve cells is reduced.
- When penetrating into the pharynx, then the trachea and bronchi, ammonia settles on the mucous membranes, dissolves, forming an alkali, which begins to have a detrimental effect on the body, causing internal burns, destroying tissues and cells.
- This substance also has a destructive effect on fatty components, which in one form or another are part of all human organs.
- The cardiovascular and endocrine systems are affected and their work is disrupted.
After contact with ammonia, almost the entire human body, its internal tissues and organs suffer, and life processes deteriorate.
Most often, cases of poisoning with this gas occur in chemical plants as a result of its leakage, but you can also be poisoned by it at home, for example, if the container containing ammonia is not tightly closed and its vapors accumulate in the room.
Poisoning can occur even when a swab soaked in ammonia is brought to the nose during a fainting state. If the victim is allowed to smell it for more than five seconds, then the risk of intoxication is high, so ammonia should always be handled with extreme caution.
How to dilute ammonia to obtain ammonia: proportions, percentages
As we have already said, it is not recommended to prepare a solution of ammonia. Yet there is a way to do it. Typically, this product is used in everyday life to get rid of insects or eliminate unpleasant odors in the kitchen. To do this, mix 1 part ammonia with 9 parts water. In percentage terms, it looks like this - 10% alcohol and 90% water.
A more complex method is somewhat reminiscent of preparing medical alcohol. The only difference is in the number of components.
How to make ammonia yourself?
The substance is prepared from vodka. But it’s not enough to just buy it and freeze it. This will not give the desired result, and therefore you will need a moonshine still. Of course, after using it you will get something stronger than vodka, but still not pure alcohol. To obtain it, distillation of vodka is required.
For this you will need:
- Confectionery yeast
- Simple package
- Glass jar
You should have everything at your fingertips. So, the preparation will be as follows:
- Pour the juice or fruit drink you prepared into a glass jar
- Add yeast to it. Expect that 30 g of yeast is added per 1.5 liters of drink
- Mix the mixture well and close the lid.
- Place the closed jar on the battery
- Open it every day to allow carbon dioxide to escape. If you don’t do this, then don’t be surprised that your jar explodes
- Do this for several days until the alcohol is released. The longer you keep the jar, the more alcohol will be released.
- After some time, the mixture will harden and the alcohol can be drained off.
To make sure that you definitely have ammonia, pour a little liquid into a tablespoon and try to heat it from below. It should flare up.
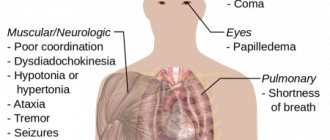
Symptoms of poisoning
Below are a number of signs of ammonia poisoning:
- Severe cough, difficulty breathing.
- Burning in the eyes, tearing, painful reaction to bright light.
- Burning in the mouth and nasopharynx.
- Dizziness, headache.
- Abdominal pain, vomiting.
- Reduced hearing threshold.
- With more serious poisoning, the following are possible: loss of consciousness, convulsions, respiratory arrest, acute heart failure. The combination of violations can lead the victim to a coma.
Using alcohol
The main areas of use of ammonia are medicine and industry. Various cleaning products and bleaches are made based on NH 4 OH. Today, when synthetic and technical materials are widely used in everyday life, the need for the use of ammonia has only increased.
In agriculture, various fertilizers made using ammonia are used. Regular feeding based on NH 4 OH allows you to provide plants with nitrogen and other microelements they need. Also, with the help of such compounds, insecticides and means for killing garden and household insects are produced.
Lotions based on ammonia perfectly relieve joint pain, reduce inflammation and help with chronic bone diseases. Recent studies have proven the effectiveness of this remedy for fungal diseases of the nail plates. External use of drugs based on the drug helps with myalgia, various nervous disorders, headaches and other pathologies.
Ammonia monohydrate is used in cosmetology, where ointments and lotions for dry skin, as well as hair rinses, are made on its basis.
Prevention in case of poisoning
First aid in this case consists of several simple steps. First, you need to take the victim out into the fresh air and wash his face and eyes with running water. Even those who were not very good at chemistry know from school: alkali is neutralized by acid, so the mouth and nose must be rinsed with water with the addition of lemon juice or vinegar.
If the poisoned person has lost consciousness, you should lay him on his side in case of vomiting, and if his pulse and breathing stop, give him a cardiac massage and artificial respiration.
Effect on the body: is the substance dangerous or not?
Is there any benefit?
There is no established benefit for the human body from the ammonium hydroxide supplement.
Possible harm and precautions
Important! If you inhale ammonia vapor for a long time, a person can get a burn to the respiratory tract, so solutions should be handled carefully, stored in tightly closed containers, when working with them, you need to wear a special mask and protect your eyes with goggles.
Ammonia is an active irritant that can negatively affect the mucous membranes of the digestive tract and disrupt the functionality of the liver and kidneys. Therefore, you should not exceed the maximum permissible dosage, and at the first signs of discomfort you should immediately seek medical help.
Do not forget that ammonium vapor in high concentrations is flammable and explosive . Fires caused by ammonia are most effectively extinguished with foam from a fire extinguisher.
Acceptable daily intake
E527 is added to products according to technical production instructions, often in combination with other hydroxide additives. For the food industry in cocoa and chocolate, the E527 norm should not be more than 70 grams per 1 kg. In other industries, the figure has been reduced to 50 g per 1 kg.
Consequences of poisoning
After ammonia intoxication, a person can face very serious irreversible consequences. First of all, the central nervous system suffers, which entails a number of complications:
- The brain ceases to fully perform its functions and begins to malfunction, because of this, intelligence decreases, mental illness, amnesia, and nervous tics appear.
- The sensitivity of some parts of the body decreases.
- The functioning of the vestibular apparatus is disrupted. Because of this, a person feels constant dizziness.
- The hearing organs begin to lose their functionality, which leads to deafness.
- When the eye covers are damaged, vision and its acuity are reduced; in the worst case, the victim will experience blindness.
- The onset of death. It depends on how high the gas concentration in the air was and how much ammonia vapor entered the body.
Knowing and following the prescribed safety measures means protecting yourself from the risk of a threat to your own life or a worse fate - disability, loss of hearing or vision.
Precautionary measures
It is necessary to exercise extreme caution when using the product and not to ignore personal protective equipment. The safety of using ammonia will largely depend on following the simplest rules:
- Ammonia hydroxide should be diluted in air or in a well-ventilated area;
- you need to avoid getting ammonia on your facial skin;
- store the drug in a locked place and out of reach of children;
- inhaling ammonia vapor is prohibited for people who suffer from vegetative-vascular dystonia;
- Do not mix alcohol with other substances.
When using ammonia as a fertilizer and plant food, you should use rubber gloves and a special protective mask. If for any reason undissolved ammonia hydroxide gets ingested, you should drink a large amount of water, induce vomiting, and then consult a doctor for help.