Ammonia gained its popularity due to its pungent odor, which helps to recover from fainting.
The pharmacy name for ammonia is ammonia solution. Why is ammonia good? Its use in medicine and in everyday life is equally widespread and useful. The area of home use for ammonia is simply enormous: it is used to clean silver, wash windows, remove stains from clothes, shoes and furniture, and is used as an insecticide. Ammonia washes glossy surfaces without streaks, and it also fights foreign odors in the refrigerator. At the dacha, it is used to make universal fertilizer.
Ammonia in medicine
The most common use of ammonia is as an analeptic (to bring one to life during a short-term loss of consciousness) in case of severe intoxication with alcohol, to treat mycosis of the nails, and to treat insect bites. Surgeons use it to disinfect their hands before operations.
- This is possible due to the caustic alkaline properties of ammonia.
- Ammonia in modern medicine is a 10% solution of ammonium hydroxide.
- Ammonia is known for its ability to stimulate the respiratory and vasomotor centers of the brain, due to its pungent and not very pleasant odor. If you overdo it, you can cause respiratory arrest. For centuries, ammonia has been used as a remedy for fainting.
- When treating bronchitis, ammonia-anise drops are sometimes prescribed as an expectorant.
- People who suffer from bronchitis are recommended to take ammonia-anise drops (as an expectorant)
- Ammonia can be a solution to such a specific problem as prolonged sneezing. The body's protective reaction to colds can vary greatly. Sometimes sneezing can become a debilitating ordeal for a person, where the person cannot stop sneezing for 10 minutes. If you give a sneezing person a sniff of ammonia, he will calm down and be able to breathe peacefully.
- Ammonia also helps with a runny nose. Since you will have to use it more than once, it is better to prepare the solution in a special way: fill a small bottle of ammonia with alcohol, leaving a few millimeters of space on top and add vegetable oil. Thus, the oil will cover the ammonia from evaporation, preserving it. Shake the bottle before use.
- If you experience a runny nose or obsessive sneezing, you need to bring the open bottle to each nostril in turn, pinching the other part of the nose. You should not immediately take deep breaths, you may get a feeling similar to a blow.
There is a mixture of ammonia that is great for headaches. To make it, you need to mix 100 ml of pharmaceutical ammonia and 10 ml of camphor. The mixture mixes quite poorly, forming white flakes, so it’s easier to close the lid and shake the vessel for a long time. This mixture is suitable for compresses against headaches. This same remedy will help with joint pain. If you lubricate your ear with the mixture and put a tampon inside the shell, this will help with ear pain. A moistened cotton wool applied to a sore tooth will help relieve discomfort.
- For joint pain, you can prepare a more specific remedy.
- Mix a spoonful of ammonia and three tablespoons of water with flour. Apply the resulting dough to the sore spot. Pain from osteochondrosis will be soothed by rubbing with tincture of ammonia and red pepper 5:1.
- For polyarthritis, lotions are prepared from honey, ammonia, iodine, glycerin and medical bile. It should be applied to the affected area for a day.
- Bronchial asthma is relieved by drinking milk with a few drops of ammonia. You need to drink it for a month before meals. During attacks, you can drink this warm milk every 2 hours until the cough calms down.
Ammonia infused with thuja with iodine helps against radiculitis. In villages, ammonia was used to get rid of binge drinking, sober up and induce vomiting.
- Ammonia mixed with glycerin in a 1:2 ratio helps with bedsores. After processing and drying, the application area should be sprinkled with baby sprinkles.
- An infusion of ammonia and dope seeds helps against endemic goiter. The tincture should be applied to the throat for 10 days and wrapped in a warm scarf.
- A mixture of 1 ml ammonium hydroxide, 2 ml acetic acid, 1 ml salicylic acid, 30 ml camphor alcohol, 5 g sulfur based on 50 ml medical alcohol helps with heel spurs.
- If you rub the barley with a small amount of ammonia, it will come off without ripening.
AMMONY
AMMONY
– the old name for ammonium chloride NH4Cl.
This salt (density 1.527 g/cm3) forms colorless crystals with an ammonia odor, highly soluble in water (37.2 g/100 g of water at 20°). Ammonium chloride melts at 400°C, but this only occurs at elevated pressure. Under normal conditions, highly volatile NH4Cl easily sublimes, and above 335°C it decomposes. Ammonium chloride does not form crystalline hydrates. Reacts with concentrated sulfuric acid, alkalis, and various oxidizing agents. Also on topic:
CHEMISTRY HISTORY
Aqueous solutions of ammonium chloride are acidic and turn blue litmus paper red due to the hydrolysis reaction:
NH4Cl = NH4+ + Cl-
Also on topic:
MEDICINE
NH4+ + 2H2O NH3 H2O + H3O+,
in which, in addition to oxonium cations H3O+ - carriers of acidity, the weak base ammonia hydrate NH3·H2O is formed. When heated, ammonium chloride sublimes with simultaneous decomposition into ammonia and hydrogen chloride:
NH4Cl NH3 + HCl
After cooling the mixture of these gases, ammonium chloride is formed again, the reaction proceeds in the opposite direction. When hydroxides of alkali and alkaline earth elements act on ammonium chloride, ammonia NH3 is released, for example, in a reaction with calcium hydroxide:
2 NH4Cl + Ca(OH)2 = 2 NH3 + CaCl2 + H2O
Ammonium chloride reacts with bromine water, oxidizing to nitrogen:
2NH4Cl + 3Br2 = N2 + 2HCl + 6HBr
At elevated temperatures, ammonium chloride reacts with salts - nitrates and nitrites, forming gaseous dinitrogen oxide and nitrogen:
NH4Cl + KNO3 = N2O + KCl + 2H2O
4Cl + KNO2 = N2 + KCl + 2H2O
These reactions occur even in solution.
In nature, ammonia does not form large accumulations. It is found in the form of small deposits and crusts, often together with sulfur, near volcanoes, in caves and cracks in the earth's crust. Ammonia was known to the priests of Ancient Egypt as far back as one and a half thousand years BC. It was obtained by sublimation from the soot of chimneys of furnaces heated by camel dung. The Arabs and Egyptians called the resulting colorless crystals “nushadir” (hence the word “ammonia”). Ammonium chloride was also a natural product of the decomposition of animal urine and feces in the oasis of Ammon in the Libyan Desert, through which numerous caravans passed. The temple of the god Ammon, widely known in Egypt, was located here. The name of the oasis was later reflected in the words “ammonia”, “ammonium salts”. Those who worshiped the god Ammon were called "Ammonians." During their rituals, they sniffed ammonia and froze in blissful ecstasy; Presumably, in those days ammonium chloride served as some kind of drug...
Alchemists who received ammonia and hydrogen chloride always observed with amazement and mystical horror the formation of white smoke when these two gases were mixed. They believed that two “spirits” met in the air, the battle of which ended with the shedding of their “blood” - the formation of smoke and a white coating on surrounding objects. In fact, it was a reaction of neutralization of a gaseous weak base - ammonia - with a volatile strong acid - hydrogen chloride.
In 1673, the English physicist and chemist Robert Boyle (1627–1691) observed a stick dipped in hydrochloric acid smoking over burning manure. The “smoke” was the result of the formation of tiny crystals of ammonium chloride when ammonia released from manure reacted with hydrogen chloride.
Until the 17th century Ammonium chloride was imported into Russia from Egypt and India. Only in 1710 was the first factory of the boyar’s son Nechaevsky built in the Yenisei province, which began to produce about five tons of ammonia per year. Ammonium chloride was obtained at this plant by neutralizing an aqueous solution of ammonia (ammonia) with hydrochloric acid.
Ammonia is used in medicine in the form of an aqueous solution as a diuretic. Ammonium chloride is easily absorbed from the gastrointestinal tract and is converted into urea (NH2)2CO with the simultaneous formation of hydrochloric acid:
2NH4Cl + CO2 = (NH2)2CO + 2HCl + H2O
Hydrochloric acid and urea are removed from the body in the urine, carrying away excess water, and at the same time sodium chloride ions, which cause edema.
Ammonium chloride was previously used in the treatment of bronchitis and pneumonia as an expectorant. In the old days, elderly society ladies, fearing fainting in the stuffiness, always carried bottles of “smelling salts” - ammonia. In an aqueous environment (for example, under the influence of moisture absorbed from the air), ammonium chloride undergoes hydrolysis, so it always smells a little like ammonia. Inhalation of ammonia causes stimulation of the respiratory centers. In small amounts, ammonia is a first aid remedy that helps bring a person out of fainting, however, large amounts of ammonia can cause respiratory arrest.
Ammonium chloride can be found in dry cell batteries or voltaic cells and is used in soldering and tin-plating of steel parts along with zinc chloride. The volatility of ammonium chloride is a property that allows the use of ammonia as a component of smoke-forming compositions. The fertilizer made from ammonium chloride is not very good (the nitrogen content is low, and the chloride ion is harmful to plants). But in medicine, ammonium chloride is still used (indispensable for edema, enhances the effect of diuretics - diuretics).
Lyudmila Alikberova
Why do you need ammonia in the garden?
In the garden, ammonia is not only a fertilizer and top dressing, but also an excellent pest repellent.
Dilute 50 ml of ammonium hydroxide in 4 liters of water and fly your shrubs to the roots. This will not only increase fertility, but also protect the roots from mole crickets. If you spray the leaves, you can fight aphids. For indoor flowers, the use is similar, but you should slightly reduce the dose.
The benefits of ammonia in the household
Ammonia has long been known as a stain remover, especially in areas where work needs to be done without streaking. If you add a few drops of ammonia when cleaning the floor, the smell will make insects leave the house. This technique can be used not only at home, but also outdoors. Spraying an ammonia solution can repel both mosquitoes and midges.
Ammonia is perfect for adding shine to crystal. If you wipe glass surfaces with a drop of ammonia, even stubborn stains will come off.
The unpleasant odor of ammonia tends to disappear quickly due to the high volatility of the substance.
Therefore, no matter how contradictory it may seem, ammonia is an excellent remedy for unpleasant odors in the home. It is worth leaving dishes with ammonia in the room for several days, then thoroughly checking and there is no trace of unpleasant odors.
What is the difference
Ammonia is one of the compounds of nitrogen with hydrogen, in which the molecule consists of three hydrogen atoms and one nitrogen atom NH3.
From the history of discovery. AM was obtained in 1774 by the English scientist D. Priestley. And in 1785, the Frenchman C. L. Berthollet, famous for the discovery of berthollet salt, determined the formula of the new gas and came up with a name.
Expert opinion
Karnaukh Ekaterina Vladimirovna
Graduated from the National University of Shipbuilding, majoring in Enterprise Economics
Under normal conditions, the NH3 compound, that is, AM, is a gas with an unpleasant and pungent odor. Ammonia is, as a rule, a 10% solution of ammonia hydrate in water, that is, it is a liquid. It is completely wrong to call such a solution ammonia. Because ammonia is a salt obtained by the reaction of ammonia and hydrochloric acid, that is, a solid substance.
To summarize, AM, NS, and ammonia differ in their condition, composition of the chemical compound and application.
Composition and manufacturing principle
AM and NS are widely used in consumption. Technological processes have been thoroughly developed for their production.
Ammonia production
AM is a chemically active gas, lighter than air. It liquefies well at a temperature of -33 °C and is easily soluble in water. It can be obtained industrially and laboratory methods.
When producing large volumes of AM for sectors of the national economy, it is obtained in gaseous form. The gas is then liquefied. For consumers, it comes in the form of a 25% aqueous solution called ammonia water.
From the history. Industrial production of ammonia appeared in 1909 in Germany. Its inventor, F. Haber, received the Nobel Prize for this. In Russia, the first plant started operating in 1928.
The production of AM is called synthesis. Given its widespread use as a starting material in many fields, ammonia synthesis is an important chemical production.
Production of ammonia
NS is a colorless transparent liquid. This is a 10% ammonia water solution with the formula NH4OH. It evaporates easily and has an ammonia smell that persists when frozen.
It is produced industrially by synthesis from nitrogen gas, hydrogen and air using catalysts. The result of production is a water-alcohol solution with a characteristic odor. In everyday life, dilution of 25% ammonia water to 10% concentration is sometimes used.
Properties of liquids
The properties of ammonia are used for widespread use in medicine. It can be used for restoration and disinfection. The most famous is the irritating effect of the drug, thanks to which it is possible to bring a person out of unconsciousness. This quality is explained by a specific pungent odor that affects the respiratory and circulatory systems.
Expert opinion
Karnaukh Ekaterina Vladimirovna
Graduated from the National University of Shipbuilding, majoring in Enterprise Economics
But concentrated aqueous solutions of ammonia are a caustic substance. Their use can damage the respiratory system and human skin. Simply diluting concentrated solutions to the required degree for use can have negative consequences.
The reason for this is the following. AM solutions are technical liquids not intended for contact with the human body. They may contain harmful impurities that have an unpredictable effect on the human body.
Scope of application
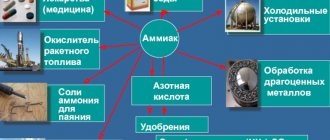
Despite its toxicity, ammonia is widely used for various purposes. First of all, this is the production of chemical products:
- ammonia and nitrate-ammonium fertilizers;
- soda ash;
- Nitric acid;
- solvents;
- explosives;
- refrigerants.
Ammonia is used in medicine:
- to relieve fainting and stimulate breathing;
- in surgery;
- to initiate vomiting, especially in case of poisoning;
- for insect bites;
- for rubbing for neuralgia.
It is no less popular in everyday life, for:
- removing stains;
- surface cleaning;
- metal cleaning;
- removing odors;
- whitening;
- as a fertilizer.
Terms of use
The materials in question are dangerous if used incorrectly. Therefore, when using them you need to adhere to certain rules.
In medicine
For normal use, NS is available in glass vials or ampoules, which should be stored in dark places.
- To bring a person out of a fainting state, cotton wool soaked in ammonia is brought to his nostrils. It should be kept at a short distance to avoid burns to the mucous membrane.
- In case of alcohol poisoning, give a solution orally at the rate of 5-6 drops per glass of water.
- To provoke vomiting, give a solution of 5-7 drops in half a glass of water.
- For insect bites, apply lotions at the site of the bite.
In agriculture
Fertilizers based on AM are produced in industrial quantities and are called nitrogen fertilizers; they are widely used. The most popular of them are ammonium sulfate and ammonium nitrate.
Ammonium sulfate contains nitrogen content up to 22%. Fertilizer is sold in granules, but it is most effective to apply it in the form of an aqueous solution. Ammonium nitrate has several varieties with their own characteristics. Among them:
- simple;
- saltpeter B;
- potash;
- calcium;
- limestone;
- sodium;
- magnesium
Each of them is used according to the instructions for use.
Fertilizing plants with ammonia is also popular. For indoor plants, use a root application with a solution in the ratio of 25 ml of alcohol per 2 liters of settled water. To combat midges and aphids, plant leaves are wiped with a solution obtained from 50 ml of alcohol diluted in 7 liters of water. The smell of the liquid repels insects.
The effect of ammonia solutions on humans
If used incorrectly, the substances discussed can adversely affect the human body. Keep in mind:
- If you inhale HC vapors or AM solutions in large quantities, your heart rhythm may be disrupted and your breathing may even stop.
- If NS is taken orally in significant doses, indigestion, abdominal pain, vomiting and cramps will occur.
- Excessive external exposure causes skin burns.
- Overdose by inhalation causes cough, runny nose, swelling of the larynx, and possible respiratory arrest.
Can you be poisoned by ammonia?
No matter how common ammonium hydroxide is, ammonia is dangerous to human health. People suffering from epilepsy should never snort ammonia. Instead of relieving the condition, this will cause a resumption of seizures. Do not use products with ammonia during exacerbations of skin diseases.
You should not use ammonia-based products on children - it is likely to cause more poisoning than in an adult. Inhalation of ammonia vapor can cause severe poisoning. Ammonia poisoning can be identified by coughing, severe shortness of breath, and a feeling of heaviness in the chest. If you feel a spasm in the larynx and you have been in contact with ammonia, then most likely you are poisoned by ammonia. In severe poisoning, hallucinations and swelling of the respiratory system may occur.
If ammonia vapor gets inside, you can expect diarrhea with blood, a drop in pressure, and pain in all areas of the gastrointestinal tract that ammonia has reached. The victim requires immediate hospitalization.
Undiluted ammonia solution may cause burns if it comes into contact with skin. In this case, this area needs to be rinsed for a long time.
If you feel slightly unwell from contact with ammonia, drink milk, egg white or a boatful of butter.