An aqueous solution of ammonia or ammonia is a universal remedy used in medicine, everyday life and gardening. A colorless liquid with a strong, pronounced odor has disinfecting properties, it can be used in agriculture as a fertilizer, is indispensable in the production of plastics and is a component of various synthetic materials.
Origin story
The prototype of ammonia alcohol was invented in Ancient Egypt, where ammonia was isolated from camel excrement. After the simplest processing, it turned into a substance whose chemical formula and properties were almost similar to modern ammonia. Subsequently, the production method, properties and formula of ammonia were changed many times, which made it possible to obtain a concentrated saturated solution, characterized by versatility of use.
In ancient times, an aqueous solution of ammonia was used for medical purposes. With the help of such a remedy, people were brought back to their senses when they fainted, instruments were disinfected during simple operations, and nervous disorders were treated. Ammonia received its name due to the components it contains.
Back in the 8th century AD, scientist George Prisley found a way to make ammonia in the form of a gas. After 150 years, the exact formula of this solution was compiled, according to which it is still produced to this day. The technology for creating ammonia in solid form and powder has emerged. It was at this time that an aqueous solution of ammonia began to be called ammonia.
Biological role
Ammonia is a substance formed in the organisms of living beings during metabolism, which is a product of nitrogen metabolism in them. It plays an important role in animal physiology, but it is highly toxic to organisms and is almost never found in them in its pure form. Most of it is processed by the liver into a harmless substance - urea or, as it is also called, urea.
It also helps neutralize acids entering the body with food, maintaining the acid-base balance of the blood.
Ammonia is an important source of nitrogen for plants. They mainly absorb it from the soil, but this is a very labor-intensive and inefficient process. Some plants are able to accumulate nitrogen contained in the atmosphere with the help of special enzymes - nitrogenases. After which they process nitrogen into useful compounds, such as proteins and amino acids.
Chemical formula
Ammonia, the chemical formula of which is NH 4 OH, is a clear, colorless liquid. The solution is obtained using a special technology at high temperatures by catalyzing hydrogen and nitrogen in the air. The basis of ammonia is ammonia, which is subjected to repeated processing.
Depending on the production method used, the properties and composition of ammonia alcohol can vary significantly, which determines the scope of use of this product. In medicine and industry, concentrated formulations are indispensable, which can be additionally diluted with pure distilled water before use.
The difference between ammonia and ammonia and hydrogen nitride is the state of aggregation of these two compounds. Accordingly, their scope of use and chemical formula differ. Ammonia is a colorless gas that liquefies at around -33°C. Ammonia is a liquid obtained by catalyzing nitrogen and hydrogen.
Ammonia monohydrate, which has a chemical formula and properties similar to ammonia, is used primarily as a medical antiseptic. This solution is also well known to housewives who use it to remove stains and feed indoor flowers.
Final table
And for ease of understanding how the materials considered differ, we offer a summary table:
| Substance | State | Chemical compound | Formula | Application | Properties |
| Ammonia | Gas | Hydrogen nitride | NH3 | Industry, fertilizers | No color. Strong smell. |
| Ammonia | Liquid | Ammonium hydroxide | NH4OH | Medicine, fertilizers | No color. Strong smell. |
| Ammonia | Solid | Ammonium chloride | NH4Cl | Fertilizers, food additive, flux | Odorless powder, white |
Basic properties
The scope of use and properties of ammonia will vary significantly depending on the specific type of drug. The formula of ammonia in chemistry is unchanged, but in reality there are numerous varieties of this drug, which may consist of different concentrations of ammonia. The solution has the following properties:
- when used externally, it perfectly regenerates tissue and improves the outflow of metabolites;
- has pronounced antiseptic properties;
- blocks nerve signals, which makes it possible to consider it as a simple pain reliever;
- when used topically, releases kinins and prostaglandins;
- improves vascular tone, stimulates heart activity;
- reduces muscle spasms;
- improves blood flow and increases blood pressure;
- has a slight calming effect;
- promotes the removal of sputum;
- stimulates the secretion of glands when used internally.
Prevention in case of poisoning
First aid in this case consists of several simple steps. First, you need to take the victim out into the fresh air and wash his face and eyes with running water. Even those who were not very good at chemistry know from school: alkali is neutralized by acid, so the mouth and nose must be rinsed with water with the addition of lemon juice or vinegar.
If the poisoned person has lost consciousness, you should lay him on his side in case of vomiting, and if his pulse and breathing stop, give him a cardiac massage and artificial respiration.
Using alcohol
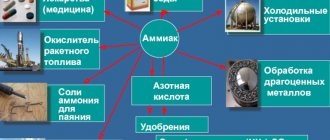
The main areas of use of ammonia are medicine and industry. Various cleaning products and bleaches are made based on NH 4 OH. Today, when synthetic and technical materials are widely used in everyday life, the need for the use of ammonia has only increased.
In agriculture, various fertilizers made using ammonia are used. Regular feeding based on NH 4 OH allows you to provide plants with nitrogen and other microelements they need. Also, with the help of such compounds, insecticides and means for killing garden and household insects are produced.
Lotions based on ammonia perfectly relieve joint pain, reduce inflammation and help with chronic bone diseases. Recent studies have proven the effectiveness of this remedy for fungal diseases of the nail plates. External use of drugs based on the drug helps with myalgia, various nervous disorders, headaches and other pathologies.
Ammonia monohydrate is used in cosmetology, where ointments and lotions for dry skin, as well as hair rinses, are made on its basis.
Instructions for use
Ammonia is not a medicine, so it must be used carefully and only as part of complex therapy. The solution is used in the following ways:
- inhalations stimulate breathing and bring a person out of fainting;
- external use for disinfection of medical instruments and hands during surgical operations;
- ingestion to trigger the gag reflex.
To bring a person who has fainted back to consciousness, it is necessary to moisten a cotton swab with an ammonia solution and bring it no closer than 5 cm to the nose. It must be remembered that close inhalation of the ammonia solution is prohibited, since a high concentration of vapors can lead to burns of the nasal mucosa.
In case of insect bites and a severe allergic reaction, it is necessary to make lotions from ammonia. They are performed either with an undiluted solution or diluted with boiled water. This reduces inflammation, disinfects the wound, reduces the allergic reaction and prevents the occurrence of anaphylactic shock.
Using a solution of ammonia, you can induce vomiting and cleanse the stomach, which is necessary in case of poisoning of the body. To do this, you will need to dilute 10 drops of ammonia in 100 ml of warm water. To induce vomiting and clear the stomach, the patient needs to drink the prepared solution.
For a wet, persistent cough, inhalations with an ammonia solution are effective. Such procedures are performed using a special nebulizer device and are prescribed exclusively by the attending physician. Several inhalations are enough to relieve a cough or completely eliminate it, while simultaneously improving the patient’s general condition.
When using ammonia for household or medical purposes, you must remember that it is a toxic substance that, if used incorrectly, can cause burns, lead to reflexive cessation of breathing and other health problems. With prolonged exposure to ammonia on the body, inflammatory and necrotic changes appear in the tissues.
Before using an ammonia solution, you should consult a specialist and read the instructions in detail. If the container containing the drug is damaged, be sure to ventilate the room, and if ammonia gets on the skin or mucous membranes, rinse the affected areas with running water.
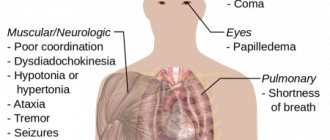
Symptoms of poisoning
Below are a number of signs of ammonia poisoning:
- Severe cough, difficulty breathing.
- Burning in the eyes, tearing, painful reaction to bright light.
- Burning in the mouth and nasopharynx.
- Dizziness, headache.
- Abdominal pain, vomiting.
- Reduced hearing threshold.
- With more serious poisoning, the following are possible: loss of consciousness, convulsions, respiratory arrest, acute heart failure. The combination of violations can lead the victim to a coma.
Precautionary measures
It is necessary to exercise extreme caution when using the product and not to ignore personal protective equipment. The safety of using ammonia will largely depend on following the simplest rules:
- Ammonia hydroxide should be diluted in air or in a well-ventilated area;
- you need to avoid getting ammonia on your facial skin;
- store the drug in a locked place and out of reach of children;
- inhaling ammonia vapor is prohibited for people who suffer from vegetative-vascular dystonia;
- Do not mix alcohol with other substances.
When using ammonia as a fertilizer and plant food, you should use rubber gloves and a special protective mask. If for any reason undissolved ammonia hydroxide gets ingested, you should drink a large amount of water, induce vomiting, and then consult a doctor for help.
Ammonia hydroxide cost
The cost of ammonia in pharmacies will depend on the concentration, volume of the ampoule or bottle, as well as the type of this substance. On average, today the price of ammonia hydroxide ranges from 15 to 60 rubles per 40 ml bottle.
The most popular is inexpensive 10% ammonia, which is available in ampoules, bottles and bottles. There is no difference in the quality of domestic and imported drugs, so most buyers prefer inexpensive products from Russian pharmacological companies.
How do you like the article?